
They will be having Maximum authority due to the presence of two grooming groups and they will be showing negative. Oh the substitution that are linked to each compound, we can write the increasing order increasing order paul activity for the above compound as we will be having compound B. We have seen double bond here and always this is labeled as compounding now according to the according to the inductive effect.
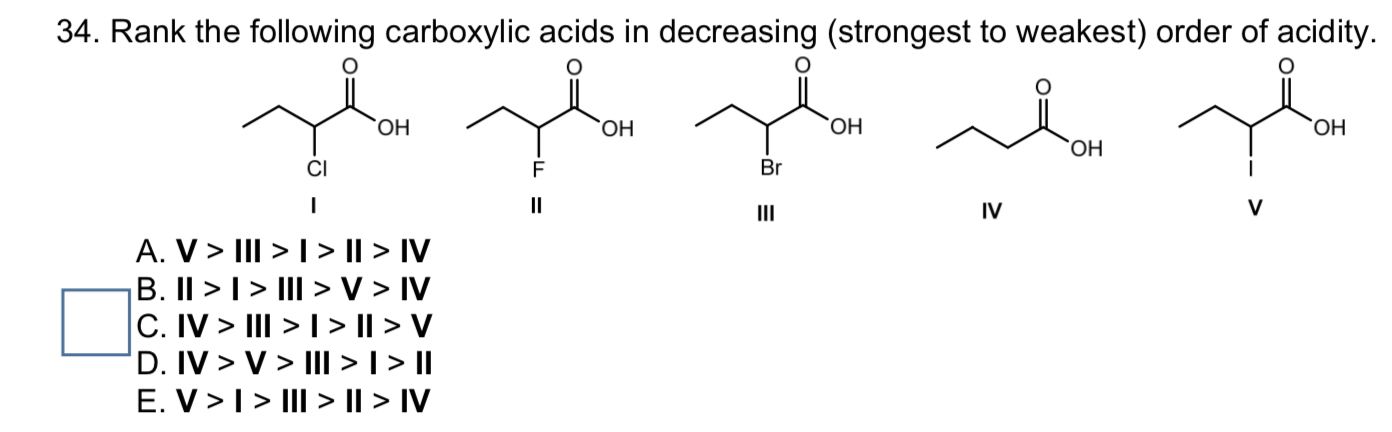
The best physical activities for men according to their age. There are two bromine atoms linked together. A I> II> III B III> II> I C II> III> I D II> I> III. Arrange the following compounds in order of increasing acidity from 1 (weakest acid) to 4 (strongest acid) A: CH3CH2COOH (propionic acid) B: CH3CFHCOOH (2-fluoropropionic acid) C: CH3CHCICOOH (2-chloropropionic acid) D: CF3CH2OH (trifluoroethanol). And moving on to the structure of the last some pounds On the first carbon. Double Bondo which and onto the veg bond here we have ch three group. Therefore it increases electron density as a result removal of proton becomes difficidt. Incase of acetic acid, methyl group is electron doner. Chlorine is electronegative element, it attracts the electrons towards itself, as a result removal of proton from chloroacetic acid becomes easier.
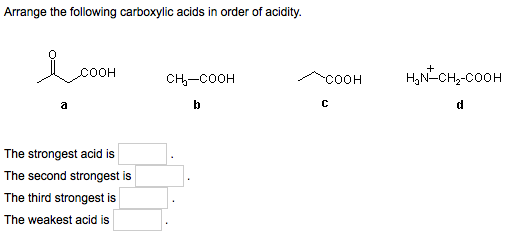
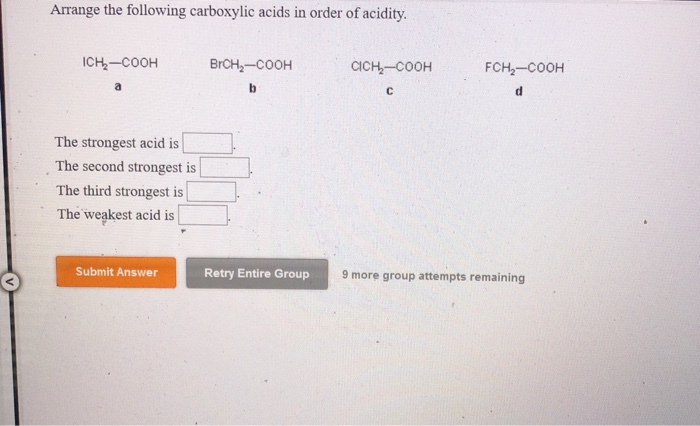
Oxygen atom is electronegative and exerts electron withdrawing inductive effect (I effect). Acetic acid < Fromic acid< Chloroacetic acidProton donors are acids. Moving on to the structure of the second compound that we have here we have double bond poet basically all the compounds are carb oxalic acid groups, compounds they contain the groups of delicacy. Increasing order of their acidic strength: 2 > 3 > 1. This is the structure of the first compound here we have a witch labor last compound and on the second carbon we have Sinai group linked towards the dash bond. In the following examples, an attempt has been made to arrange the acids in decreasing order of acidic strength. In this question we are given with views of compound for which we have to write the correct increasing order of authority of the compound. Arrange the following carboxylic acids in order of increasing acidity : m. The acidic strength of substituted carboxylic acid is known to depend on nature and position of substituents.


 0 kommentar(er)
0 kommentar(er)
